Treatment of systemic lupus erythematosus with umbilical cord stem cells
An abnormal immune response that causes inflammation and damage to target tissues or organs, such as the skin, kidneys, joints, and muscles, causes systemic autoimmune diseases [1]. Systemic lupus erythematosus (SLE) is a multisystem, multiorgan-damaging, and potentially fatal autoimmune disease characterized by the activation and proliferation of T cells and B cells [2]. Due to clinical heterogeneity and unpredictable disease recurrence, SLE treatment is challenging. Several medications have undergone research, but they commonly result in various side effects during and after the cessation of the treatment course. MSC transplantation has emerged as a potentially beneficial therapy for autoimmune diseases, especially SLE, based on its strong immunosuppressive ability.
SLE treatment using corticosteroids or anti-inflammatory pain relievers
Systemic lupus erythematosus (SLE) is a late-stage systemic autoimmune disease that often causes many complications to organs, including the heart, lungs, kidneys, nerves, and hematopoietic system. Depending on the complication symptoms, we can use anti-inflammatory drugs such as Ibuprofen, Aspirin, prednisone, chloroquine, methotrexate, tacrolimus, and azathioprine, which have significantly improved the survival of SLE patients [3]. However, they often cause side effects such as stomach ulcers, osteoporosis, increased risk of infection, and bone marrow suppression, and the disease can recur after stopping the drug [4]. Conventional therapy often requires the use of multiple immunosuppressive medicines for several years or even the patient’s lifetime, which not only affects the patient’s life quality but also aggravates the financial and psychological burden. Therefore, we still need more effective and safer therapies in the future. One of those therapies is the use of mesenchymal stem cells (MSCs) based on the immunomodulatory and regenerative properties of MSCs. This may be a better choice for patients who respond poorly to conventional therapy. MSC therapy has shown positive efficacy with tolerable treatment-related side effects and is a safe and effective therapy [5]. The properties and therapeutic effects of MSCs make them a promising therapy in the treatment of SLE.
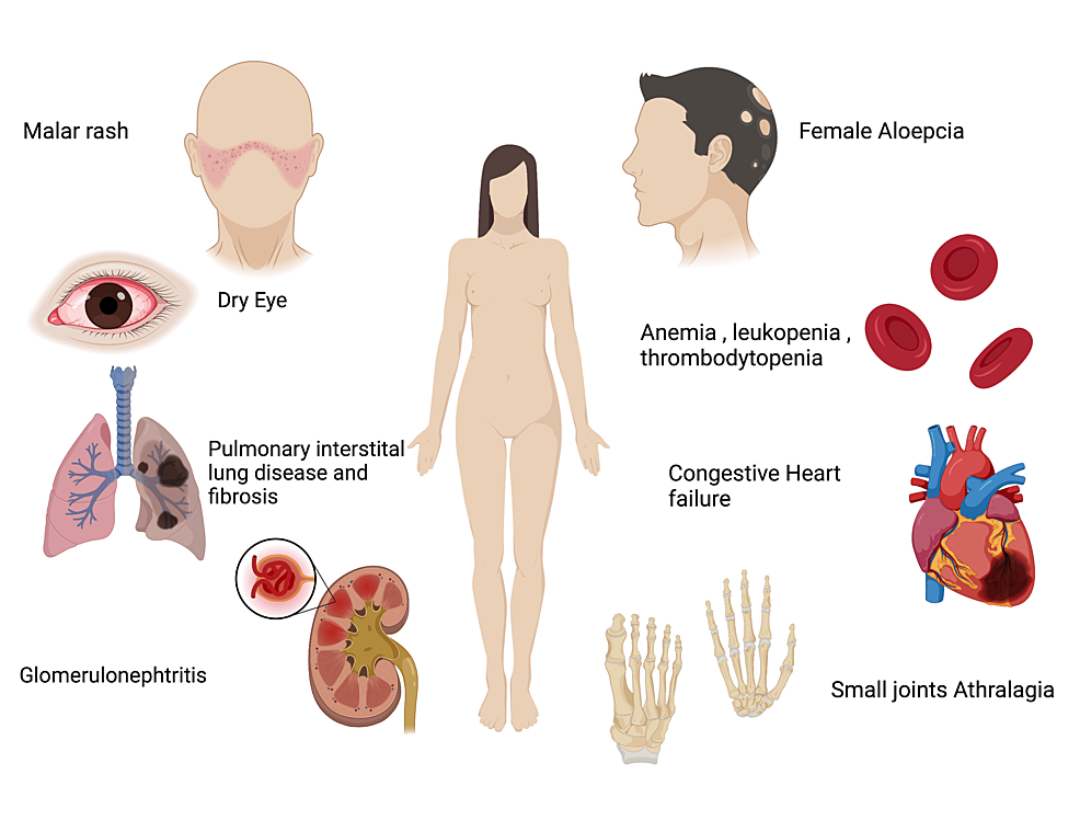
Advantages and prospects of SLE treatment using MSCs
MSCs are multipotent, self-renewing stem cells that can differentiate into various mature, specialized cells. MSCs can also regulate the adaptive immune system in patients with autoimmune diseases [6]. Allogeneic transplantation of MSCs from healthy individuals with no autoimmune disease history is highly effective in SLE patients and can improve their symptoms. In contrast, autologous MSCs in SLE patients have no immunosuppressive effect and do not improve disease symptoms because the MSCs are dysfunctional in both proliferation and immunoregulation. In addition, SLE also shows a loss of homeostatic regulation in the body because of loss of autoimmune control. The key to that problem is Tregs’s abnormal activity. Tregs act by downregulating the activation and proliferation of effector T cells [7]; allogeneic MSC transplantation can restore the balance of Tregs in SLE patients.
In recent years, the number of clinical trials for stem cell therapies for autoimmune diseases registered on the website www.clinicaltrials.gov has exceeded 200 cases, most of which are MSC or hematopoietic stem cells (HSC). Autologous HSC infusion also restores Treg numbers and immunosuppressive function in SLE patients [8]. The success of autologous HSC therapies has encouraged the use of MSCs to treat SLE patients. Several clinical studies have evaluated the potential clinical effectiveness of MSC transplantation as an alternative treatment to current pharmacotherapy for SLE.
MSCs have undergone extensive research and application in regenerative medicine and tissue engineering. Recently, their immunomodulatory functions have made them an attractive potential approach to treat autoimmune diseases. Notably, previous studies have shown that MSCs have the ability to inhibit B cell proliferation and differentiation [9] and thus may have promising efficacy in the treatment of SLE.
Clinical effectiveness and safety of MSCs from umbilical cord blood for SLE
MSCs from umbilical cord blood can be safely harvested without major ethical concerns. To date, MSCs have been successfully applied to treating many autoimmune diseases, including SLE [10]. MSCs have been studied to promote HSC engraftment and to prevent graft rejection [11]. And umbilical cord derived MSC transplantation has shown effectiveness in patients with autoimmune diseases, especially SLE [12]. MSCs from umbilical cord blood can inhibit T-cell proliferation in lupus patients by secreting large amounts of indoleamine 2,3-dioxygenase [13]. They also suppress B cell proliferation and differentiation in SLE patients. As we know, T cells are characteristically activated in SLE patients, especially those with severe types. Therefore, modulating the energy status of T cells may be a new way to treat SLE patients.
Thus, the potential to treat SLE with MSCs in general and MSCs from the umbilical cord in particular is very promising. They improve the patient’s quality of life by taking down biological and financial burdens for patients and society. However, there are still many limitations to further studying the mechanism of MSCs in SLE disease to evaluate their overall effectiveness. However, researchers still consider MSC therapy as a better hope in the treatment of SLE.
References
- Fanouriakis, Antonis, Nikolaos Tziolos, George Bertsias, and Dimitrios T. Boumpas. “Update οn the diagnosis and management of systemic lupus erythematosus.” Annals of the rheumatic diseases80, no. 1 (2021): 14-25.
- Lee, Y. H., S. J. Choi, J. D. Ji, and G. G. Song. “Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis.” Lupus25, no. 7 (2016): 727-734.
- Hahn, Bevra Hannahs. “Belimumab for systemic lupus erythematosus.” New England Journal of Medicine368, no. 16 (2013): 1528-1535.
- Bernatsky, S., J‐F. Boivin, L. Joseph, S. Manzi, E. Ginzler, D. D. Gladman, M. Urowitz et al. “Mortality in systemic lupus erythematosus.” Arthritis & Rheumatism: Official Journal of the American College of Rheumatology54, no. 8 (2006): 2550-2557.
- Wang, Dandan, Lingying Niu, Xuebing Feng, Xinran Yuan, Shengnan Zhao, Huayong Zhang, Jun Liang et al. “Long-term safety of umbilical cord mesenchymal stem cells transplantation for systemic lupus erythematosus: a 6-year follow-up study.” Clinical and Experimental Medicine17, no. 3 (2017): 333-340.
- Munir, Hafsa, and Helen M. McGettrick. “Mesenchymal stem cell therapy for autoimmune disease: risks and rewards.” Stem cells and development24, no. 18 (2015): 2091-2100.
- Li, Ming O., and Alexander Y. Rudensky. “T cell receptor signalling in the control of regulatory T cell differentiation and function.” Nature Reviews Immunology16, no. 4 (2016): 220-233.
- Zhang, Li, Anne M. Bertucci, Rosalind Ramsey-Goldman, Richard K. Burt, and Syamal K. Datta. “Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-β-producing CD8+ Treg cells are associated with immunological remission of lupus.” The Journal of Immunology183, no. 10 (2009): 6346-6358.
- Corcione, Anna, Federica Benvenuto, Elisa Ferretti, Debora Giunti, Valentina Cappiello, Francesco Cazzanti, Marco Risso et al. “Human mesenchymal stem cells modulate B-cell functions.” Blood107, no. 1 (2006): 367-372.
- Liao, Jieyue, Christopher Chang, Haijing Wu, and Qianjin Lu. “Cell-based therapies for systemic lupus erythematosus.” Autoimmunity reviews14, no. 1 (2015): 43-48.
- Gao, Lei, Yan-Qi Zhang, Jia Liu, Shi-Feng Lou, Yi Su, Tong-Hua Yang, Hui-Min Li et al. “Phase II multicenter, randomized, double-blind controlled study of efficacy and safety of umbilical cord-derived mesenchymal stromal cells in the prophylaxis of chronic graft-versus-host disease after HLA-haploidentical stem-cell transplantation.” (2016).
- Wang, Dandan, Jing Li, Yu Zhang, Miaojia Zhang, Jinyun Chen, Xia Li, Xiang Hu, Shu Jiang, Songtao Shi, and Lingyun Sun. “Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study.” Arthritis research & therapy16, no. 2 (2014): 1-14.
- Wang, Dandan, Xuebing Feng, Lin Lu, Joanne E. Konkel, Huayong Zhang, Zhiyong Chen, Xia Li et al. “A CD8 T cell/indoleamine 2, 3‐dioxygenase axis is required for mesenchymal stem cell suppression of human systemic lupus erythematosus.” Arthritis & rheumatology 66, no. 8 (2014): 2234-2245.
