Probiotic Applications in Kidney Disease Treatment
Chronic Kidney Disease (CKD) occurs due to the gradual decline in kidney function. It is characterized by the progressive reduction of organic biochemical function and secondary physiological functions due to the accumulation of metabolic waste, electrolyte imbalance, acid-base imbalance, reduced blood volume, increased blood potassium and phosphate, anemia, endocrine disorders, hypertrophy of the thyroid, infertility, and delayed development [1]. The glomerular filtration rate decreases as CKD progresses, dividing it into five stages with increasing severity. This eventually leads to end-stage renal disease (ESRD), where patients need replacement therapy, dialysis, or organ transplantation. CKD is prevalent and affects 1 in 10 individuals in the general population, with only 4 in 100,000 patients reaching the end stage [2]. Studies have demonstrated that CKD becomes more severe due to the impact of an aging population and partly due to the increasing prevalence of high blood pressure and diabetes [1].
One of the initial adverse effects of CKD is the disruption of the gut microbiota balance, leading to mucosal barrier impairment. This, in turn, exacerbates the condition of CKD patients. The gut microbiota is essential for regulating the normal function of the intestinal barrier, promoting immune defense against antigens from nutrients or organisms, controlling nutrient absorption and metabolism, and preventing the spread of pathogenic microorganisms. Therefore, dysregulation of gut microbiota may play a significant role in cancer, metabolism-related diseases, and gastrointestinal inflammation. Recently, it has been demonstrated that CKD is associated with dysregulation of the gut microbiota [3].
Challenges in CKD Treatment
Researchers have proposed several treatment methods for CKD patients, including reducing uremic toxins absorption (restricting protein intake, using adsorbents like AST-120), increasing detoxification through renal replacement therapy (dialysis, adsorption membrane, kidney transplantation), and modulating cellular pathways (organic anion transporters and antioxidant agents) [4]. Currently, kidney transplantation and dialysis are the only available treatments to control end-stage CKD. These invasive procedures have significant side effects and high societal costs, making it challenging for low-income countries to provide comprehensive treatment for kidney failure patients. Over the years, researchers have published various studies on the removal of urea waste through the gut, yielding conflicting results. Some research groups have applied probiotic bacteria’s natural biological treatment capabilities to minimize the severity in CKD patients. This evidence has opened up new prospects for CKD treatment in the context of the challenges posed by traditional methods [5].
Probiotic Mechanisms and Clinical Trials in CKD
The regenerative and regulatory effects of probiotics on the gut microbiota directly impact the progression of CKD patients (Figure 1). Probiotics exhibit antibacterial properties, forming a protective mucosal barrier by synthesizing and secreting mucus. Some strains have the ability to produce complex antibacterial compounds known as bacteriocins or antimicrobial peptides. This is particularly concerning as CKD patients have a higher risk of Clostridium difficile infection (a type of gut bacteria) compared to the general population. The primary purpose of using probiotics in CKD is to eliminate toxins and excess water from the blood. Therefore, the gut microbiota can mitigate the production of toxins, mainly resulting from protein breakdown and not completely blocked by a low-protein diet, by converting amino acids into trimethylamine N-oxide, p-cresyl sulfate, or indoxyl sulfate [6,8].
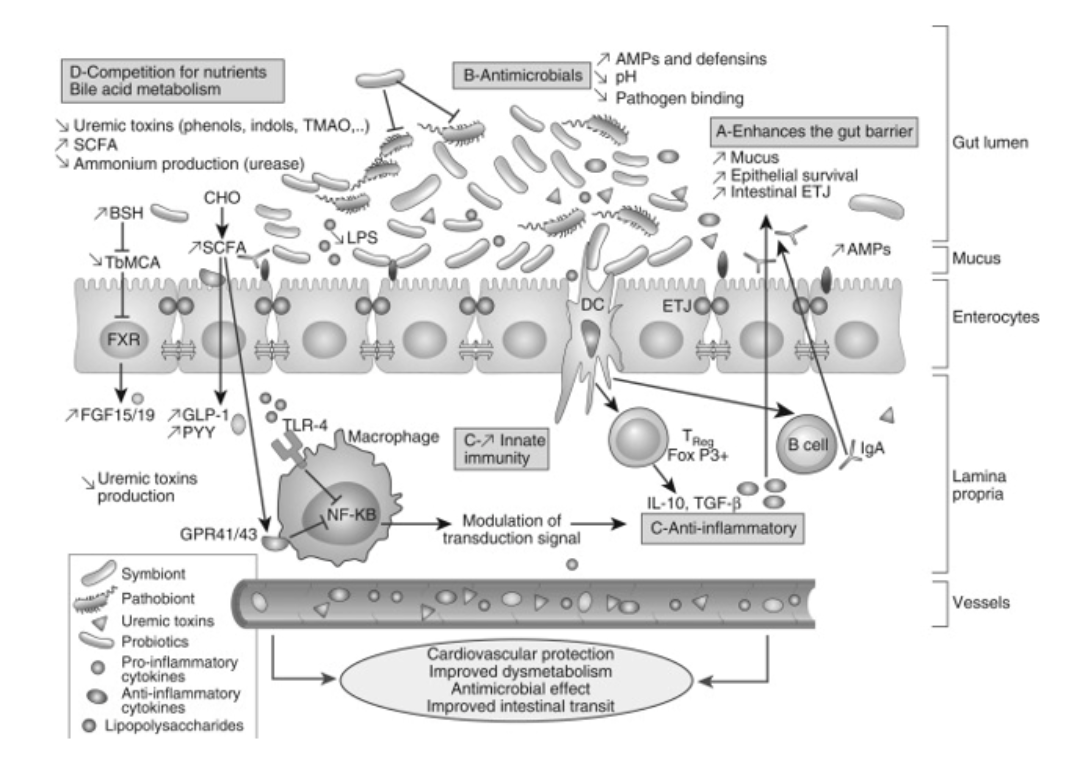
Figure 1: Overview of the overall beneficial effects of probiotics and potential effects on CKD.
Probiotics can improve cell survival and reduce programmed cell death in various gut attack scenarios. In fact, soluble factors released by Lactobacillus rhamnosus have been found to activate protein kinase β in a phosphatidylinositol-3′-kinase-dependent manner, preventing programmed cell death caused by cytokines in the intestines of humans and mice. Lactobacillus rhamnosus can produce soluble proteins (p40 and p75), protecting the gut barrier from damage caused by hydrogen peroxide. Other bioactive compounds maintain intestinal integrity by increasing tight junctions between cell layers through the regulation of zonula occludens-1 expression or by preventing tissue redistribution of binding proteins [6].
Other bioactive compounds can influence the gene expression of pathogenic microorganisms, thereby reducing their toxicity. For example, Lactobacillus acidophilus can inhibit the toxic gene expression of Escherichia coli O157:H7, which causes intestinal bleeding. Probiotics can prevent the attachment of intestinal pathogens to the mucosal surface by masking receptor-binding sites, thereby preventing pathogen invasion and allowing enhanced removal of pathogens from the digestive tract [6,10].
Other beneficial bacterial strains stimulate the innate immune system by signaling to dendritic cells, which then move to lymph nodes where they generate regulatory T cells (FoxP3+) and produce anti-inflammatory cytokines (interleukin-10 and transforming growth factor-β). For instance, Saccharomyces boulardii has been shown to reduce intestinal inflammation by modulating T-cell responses and reducing the trafficking of Th1 cells, leading to decreased pre-inflammatory interferon-γ cytokines [11].
Thanks to these probiotic features, several clinical trials have been conducted with promising results. According to Cruz-Mora J and colleagues, using a combination of probiotics (Lactobacillus acidophilus and Bifidobacterium lactis) with a prebiotic (inulin) in CKD patient treatment helps improve gastrointestinal symptoms and slow down the progression of kidney disease [9]. Another study by Laetitia Koppe and colleagues, testing 8 CKD patients treated with orally administered Lactobacillus acidophilus for 1–6 months, showed a significant reduction in dimethylamine and nitrosodimethylamine in the blood (both toxins related to increased mortality rates in CKD) [6]. The use of orally administered probiotics has proven to be safe and effective, ensuring rapid and straightforward delivery to the target organs. Lactobacillus casei, known for its strong resistance to gastric acid and bile, has been used in CKD treatment, showing good absorption and slowing the decline in kidney function in stage 3–5 CKD patients (Figure 2). The use of Lactobacillus casei through oral administration by altering SCFA and nicotinamide metabolism presents a potential approach to minimizing kidney damage and slowing the progression of kidney decline. Additionally, probiotics can improve constipation in CKD patients [3].
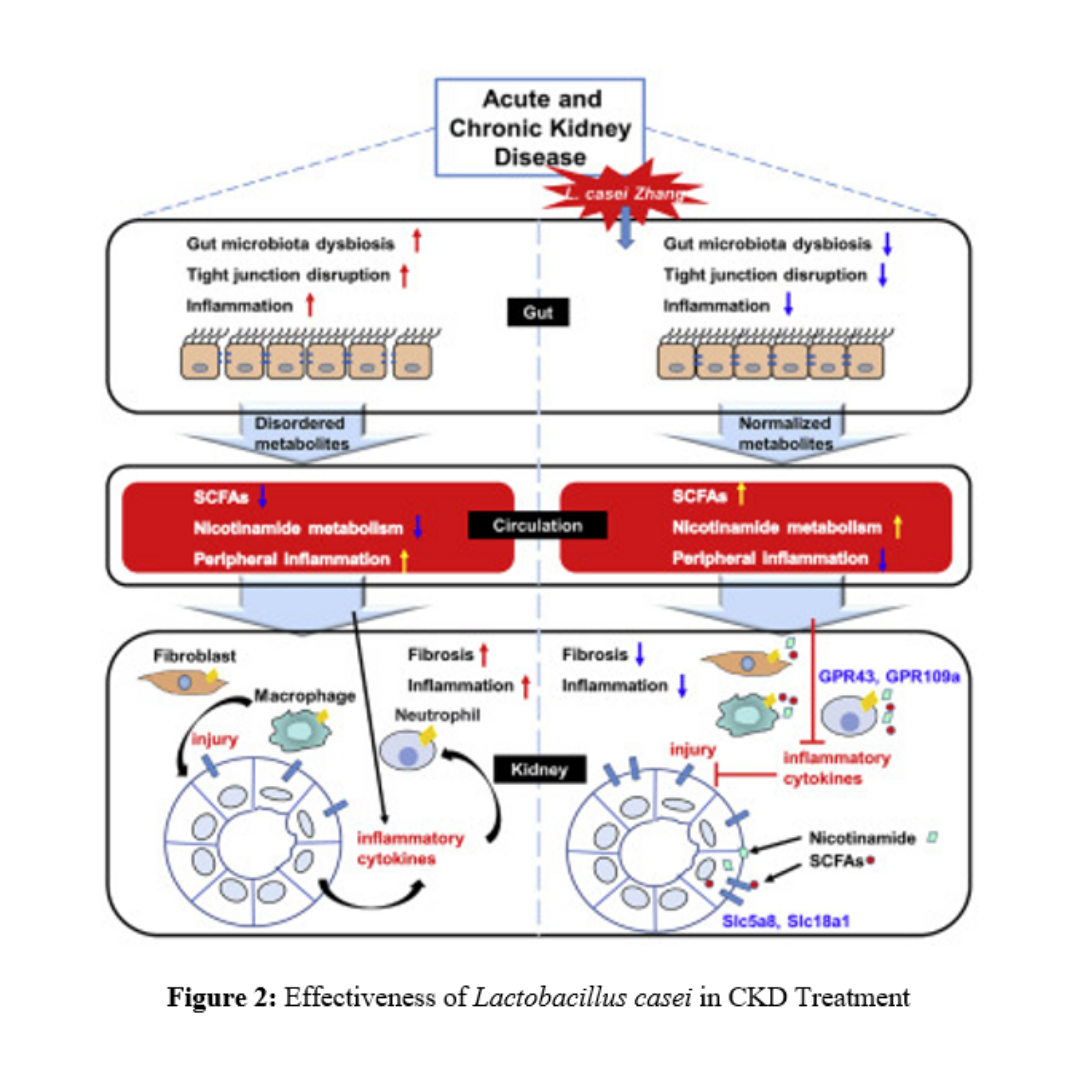
Figure 2: Effectiveness of Lactobacillus casei in CKD Treatment
Prospects of Probiotic Technology in CKD Treatment
Depending on the strain, probiotics have different fundamental mechanisms of action. The choice of a specific probiotic strain in CKD treatment is based on experience. In other diseases, methods are used to identify potential probiotics by isolating various strains of Lactobacillus, Bifidobacillus, or newly identified strains from food and systematically testing them. The convergence of potential mechanisms of action (e.g., hydrolase activity of bile salt, urease, defensins, competitive exclusion behavior against urea-producing bacteria) is currently unclear. It is also essential to note that probiotics can affect different regions of the intestine—small intestine and large intestine—each with its distinct ecosystem characteristics. Therefore, a better understanding of the microbial composition in the gut concerning its potential impact on the host is necessary to drive further exploration to select the best probiotic strains.
Furthermore, organisms may exhibit different behaviors when used as a single strain compared to when combining multiple probiotic strains. In fact, it has been demonstrated that single-cell organisms interacting with each other can enhance or inhibit the activities of other organisms. Researchers have proposed multispecies probiotic formulations for various applications and conducted studies to compare their effectiveness and differences between a single strain and multiple strains [6]. Despite this, such an approach is emerging as a treatment method for CKD.
References:
- Fagundes, R. A. B., Soder, T. F., Grokoski, K. C., Benetti, F., & Mendes, R. H. (2018). Probiotics in the treatment of chronic kidney disease: a systematic review. Brazilian Journal of Nephrology, 40, 278-286.
- Noël, D., & Landais, P. (2012). Epidemiology of chronic kidney disease. La Revue du praticien, 62(1), 38-42.
- Zhu, H., Cao, C., Wu, Z., Zhang, H., Sun, Z., Wang, M., … & Zeng, R. (2021). The probiotic L. casei Zhang slows the progression of acute and chronic kidney disease. Cell metabolism, 33(10), 1926-1942.
- Guldris, S. C., Parra, E. G., & Amenós, A. C. (2017). Gut microbiota in chronic kidney disease. Nefrología (English Edition), 37(1), 9-19.
- Di Cerbo, A., Pezzuto, F., Palmieri, L., Rottigni, V., Iannitti, T., & Palmieri, B. (2013). Clinical and experimental use of probiotic formulations for management of end-stage renal disease: an update. International Urology and Nephrology, 45, 1569-1576.
- Koppe, L., Mafra, D., & Fouque, D. (2015). Probiotics and chronic kidney disease. Kidney international, 88(5), 958-966.
- Vaziri, N. D., Wong, J., Pahl, M., Piceno, Y. M., Yuan, J., DeSantis, T. Z., … & Andersen, G. L. (2013). Chronic kidney disease alters intestinal microbial flora. Kidney international, 83(2), 308-315.
- Keddis, M. T., Khanna, S., Noheria, A., Baddour, L. M., Pardi, D. S., & Qian, Q. (2012, November). Clostridium difficile infection in patients with chronic kidney disease. In Mayo Clinic Proceedings (Vol. 87, No. 11, pp. 1046-1053). Elsevier.
- Cruz-Mora, J., Martínez-Hernández, N. E., del Campo-López, F. M., Viramontes-Hörner, D., Vizmanos-Lamotte, B., Muñoz-Valle, J. F., … & Castro-Alarcón, N. (2014). Effects of a symbiotic on gut microbiota in Mexican patients with end-stage renal disease. Journal of Renal Nutrition, 24(5), 330-335.
- Medellin-Peña, M. J., Wang, H., Johnson, R., Anand, S., & Griffiths, M. W. (2007). Probiotics affect virulence-related gene expression in Escherichia coli O157: H7. Applied and environmental microbiology, 73(13), 4259-4267.
- Dalmasso, G., Cottrez, F., Imbert, V., Lagadec, P., Peyron, J. F., Rampal, P., … & Groux, H. (2006). Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology, 131(6), 1812-1825
