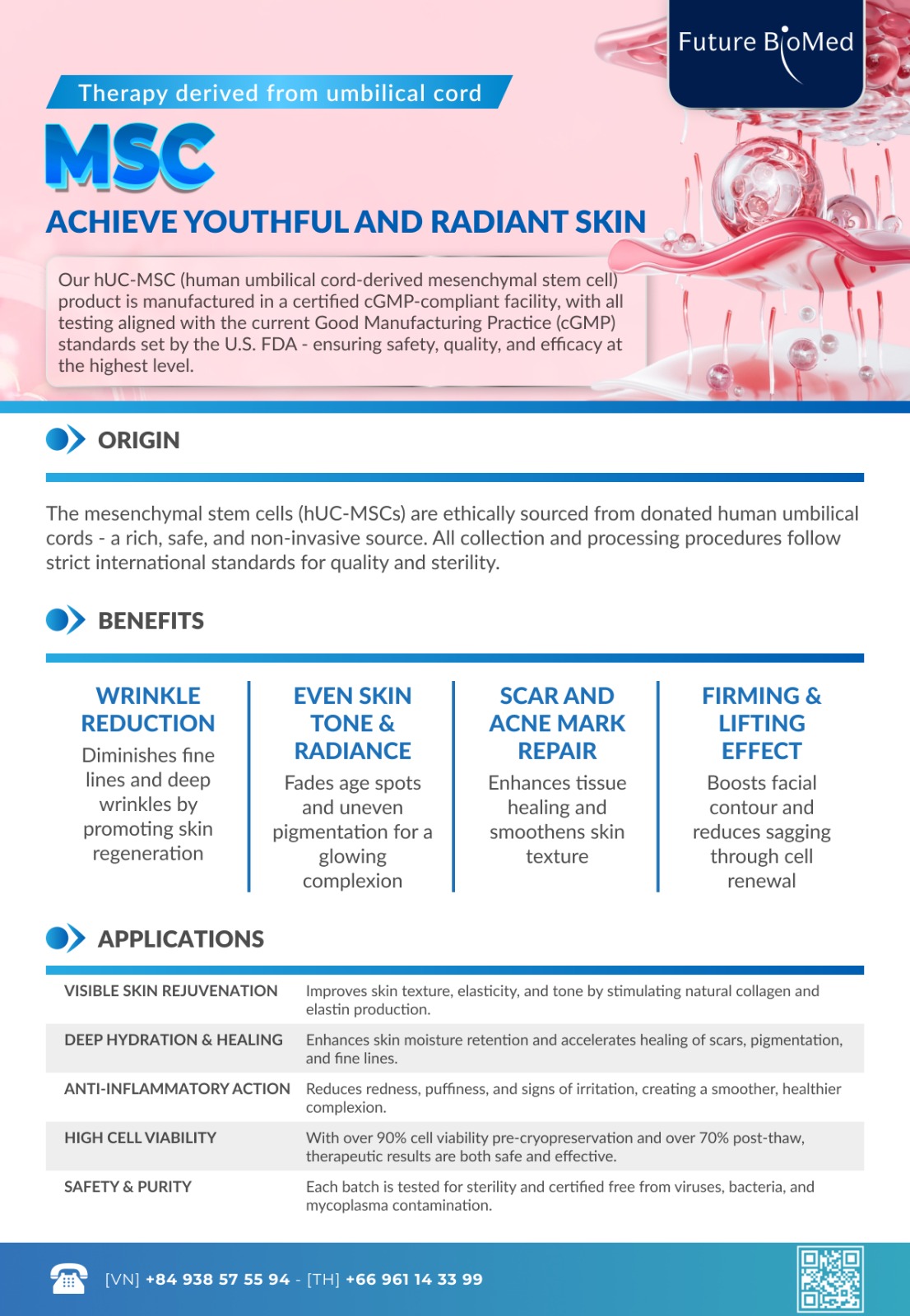
hUC-MSC BY FUTURE BIOMED
A next generation cellular solution for regenerative medicine and anti-aging
Regenerative medicine has advanced rapidly in recent years thanks to breakthroughs in biotechnology. Among all cell based therapies, Mesenchymal Stem Cells MSC stand out as one of the most important pillars. With their ability to self renew, modulate the immune system and activate endogenous tissue repair, MSCs are shaping new approaches for treatment, recovery and comprehensive anti aging.
Future Biomed provides high quality hUC MSC Cell Suspension produced under international standards. This article offers an in depth explanation of MSC biology, their clinical applications, quality benchmarks at Future Biomed and essential guidelines for storage and use of the product.
1. What are Mesenchymal Stem Cells
Mesenchymal Stem Cells MSC are multipotent adult stem cells found in various connective tissues of the body. They can self renew and differentiate into several cell lineages. Common sources include bone marrow, adipose tissue, dental pulp and especially human umbilical cord tissue.
MSC function through two key biological mechanisms.
The first is differentiation. MSCs can become osteocytes, chondrocytes, adipocytes and other types of connective tissue cells.
The second is regulation. Through the secretion of soluble factors such as exosomes, cytokines and chemokines, MSCs help reduce inflammation, guide immune cell activity, promote angiogenesis and activate the body’s natural regenerative mechanisms.
Thanks to these combined functions, MSCs are considered part of the body’s intrinsic repair system. They maintain tissue balance and support healing across different organ systems.

2. Applications of MSCs in regenerative medicine
Because of their broad biological functions, MSCs have become central to many therapeutic and research programs worldwide. Key application areas include:
2.1 Autoimmune and immune related conditions
MSCs regulate T cells, B cells, macrophages and other immune cells. This helps suppress autoimmune reactions and reduce chronic inflammation. Potential applications include lupus, rheumatoid arthritis and inflammatory bowel conditions.
2.2 Tissue injury and musculoskeletal recovery
MSC therapies support recovery from sports injuries, ligament tears, tendon inflammation, cartilage damage and soft tissue trauma. By modifying the microenvironment at the injury site, MSCs accelerate endogenous regeneration.
2.3 Degenerative diseases
Degenerative joint disorders, disc degeneration and age related tissue deterioration may benefit from MSC therapy. MSCs help improve inflammatory conditions and promote new cell formation.
2.4 Anti aging and whole body rejuvenation
One of the fastest growing fields for MSCs is anti aging. Age causes a progressive decline in cellular repair capacity. MSCs help counteract this process by:
- Reducing chronic age related inflammation
- Enhancing natural repair mechanisms
- Regulating immune function
- Supporting collagen production and skin health
- Improving hair vitality
- Slowing age related tissue degeneration
This makes MSCs a foundational tool for comprehensive cellular rejuvenation.

3. Quality standards of hUC-MSC at Future Biomed
The hUC-MSC Cell Suspension at Future Biomed is produced under a cGMP compliant process that adheres to FDA 21 CFR Parts 210 and 211. Every step is designed to meet the highest standards of safety and consistency.
3.1 Final product quality testing
Each batch undergoes strict quality testing before clinical release. Tests include:
- High cell viability that surpasses required minimum thresholds
- Sterility testing confirming no bacterial or fungal contamination
- Mycoplasma negative results validated by both Rapid and USP methods
- Endotoxin levels well below the 5 EU per mL safety requirement
- Gram stain negative confirming absence of foreign microbes
3.2 Donor safety screening
Umbilical cord tissue donors are rigorously screened. All samples test negative for:
- HBV
- HCV
- HIV 1 and HIV 2
- HTLV I and II
- Syphilis
- CMV
- West Nile Virus
In addition, donors pass a comprehensive medical history evaluation with no risk factors.
3.3 Master cell bank quality assurance
The foundational cell bank used for production is tested for:
- Verified differentiation potential
- Standardized surface marker expression
- Negative results across PCR based, in vitro and in vivo viral testing
- No viral particles detected under transmission electron microscopy
- Sterility confirmed by USP standards
These multilayer safety checks ensure that every vial of hUC-MSC supplied to Future Biomed meets strict international criteria for purity, safety and consistency.

4. Storage and handling guidelines for hUC-MSC
Proper storage and handling are critical to maintaining the biological integrity of MSCs.
4.1 Storage
The hUC MSC Cell Suspension is supplied in frozen cryovials and must be stored under the following conditions:
- Maintain temperatures below minus 150 degrees Celsius
- Store in vapor phase or liquid phase liquid nitrogen
- Avoid temperature fluctuations
- Prevent prolonged exposure to ambient conditions during transport
4.2 Thawing and preparation for use
The product should be thawed only when it will be used immediately. Correct procedure includes:
- Thawing in a controlled water bath according to SOP
- Administering the product immediately after thawing
- Never refreezing any portion of the product
- Handling within a sterile environment
- Use restricted to trained medical professionals
These steps protect the viability and safety of the MSCs before administration.

Product label sample
5. Conclusion
Mesenchymal Stem Cells play a central role in modern regenerative medicine. Their ability to self renew, regulate immune activity and activate tissue repair makes them a promising therapeutic tool for injury recovery, autoimmune conditions, degenerative diseases and advanced anti aging applications.
The hUC-MSC Cell Suspension at Future Biomed is produced according to strict international standards and undergoes extensive testing to ensure safety and performance. When stored and used correctly, MSC therapy offers a forward looking approach to health restoration and long term wellness.
Future Biomed remains committed to advancing high quality cellular therapies and providing medically innovative solutions that support sustainable health and rejuvenation.

Product label sample
📌 Learn more about this product:
hUC-MSC Cell Suspension for regeneration, immune modulation, anti-aging, tissue repair, and long-term health optimization.
For consultation and treatment inquiries:
🔻 Address: Sukhumvit Rd, Khlong Toei, Bangkok, Thailand
🔻 Bangkok hotline: 0961143399
🔻 Vietnam hotline: 0938575594
📧 Email: info.fbiomed@gmail.com
👉 Contact us today and experience the future of health and longevity.
