Natural Killer (NK) cells in cancer treatment
Cancer is a broad term for a large group of diseases that can affect many parts of the body. Other terms used are malignant tumors and neoplasms. A notable characteristic of cancer is the rapid generation of abnormal cells that grow beyond their usual boundaries and can invade neighboring parts of the body and spread to other organs; this latter process is called metastasis. Widespread metastasis is the primary cause of cancer-related deaths. Cancer is a leading cause of death worldwide, accounting for nearly 10 million deaths in 2020, which is almost 1/6th of all deaths. The most common cancers include breast, skin, lung, colorectal, prostate, and liver cancers [1].
Causes of Cancer
Cancer arises from the transformation of normal cells into tumor cells in a process involving multiple stages, often progressing from pre-cancerous lesions to malignant tumors. Causes include physical carcinogens like ultraviolet and ionizing radiation, chemical carcinogens like components of tobacco smoke, alcohol, and arsenic (a drinking water contaminant), and biological carcinogens like infections from certain viruses, bacteria, or parasites [2, 3].
Cancer burden
Cancer is an immensely dangerous disease that is increasingly prevalent over time. The cost of cancer treatment is exorbitant, and the efficacy often falls short of expectations. Avoiding risk factors and implementing evidence-based prevention strategies can prevent about 30 to 50% of cancers [2]. The burden of cancer can also be reduced through early detection, appropriate treatment, and care for cancer patients. Many cancers have a high chance of cure if diagnosed early and treated appropriately [4]. Besides preventive measures such as minimizing risks by using clean food sources, limiting alcohol and tobacco use, and increasing physical activity, scientists have been researching and starting to implement immune-based approaches to cancer treatment. Among these, scientists are giving considerable attention to natural killer (NK) cells and extensively referencing them in cancer therapy [5, 6].
Function and Mechanism of NK Cells’ action
The immune system, in general, divides into innate and adaptive immunity, both of which aid in recognizing and eliminating foreign pathogens as well as tumors. Adaptive immunity typically involves immune cells characterized by T and B lymphocytes containing numerous T-cell and B-cell receptors, thus allowing them to mount immune responses against various cells in the body [5]. Current immune therapies mainly focus on T lymphocytes. In recent years, the role of the tumor immune microenvironment in the early stages has garnered increasing interest. Researchers have identified NK cells as a potential target for cancer therapy, considering their pivotal role in cancer biology. Numerous studies and therapeutic agents have utilized pathways related to targeting NK cells for cancer treatment. As a critical player in the innate immune system, NK cells hold promise for clinical application. In recent years, studies on NK cell-based therapies have rapidly advanced, with the latest research focusing on cytokines, monoclonal antibodies, intracellular signaling pathways, cultivation processes, and genetic engineering of NK cells [6]. Furthermore, NK cell-based therapy has shown favorable outcomes when used alone or in combination with other therapies, demonstrating widespread and effective use in malignant tumors.
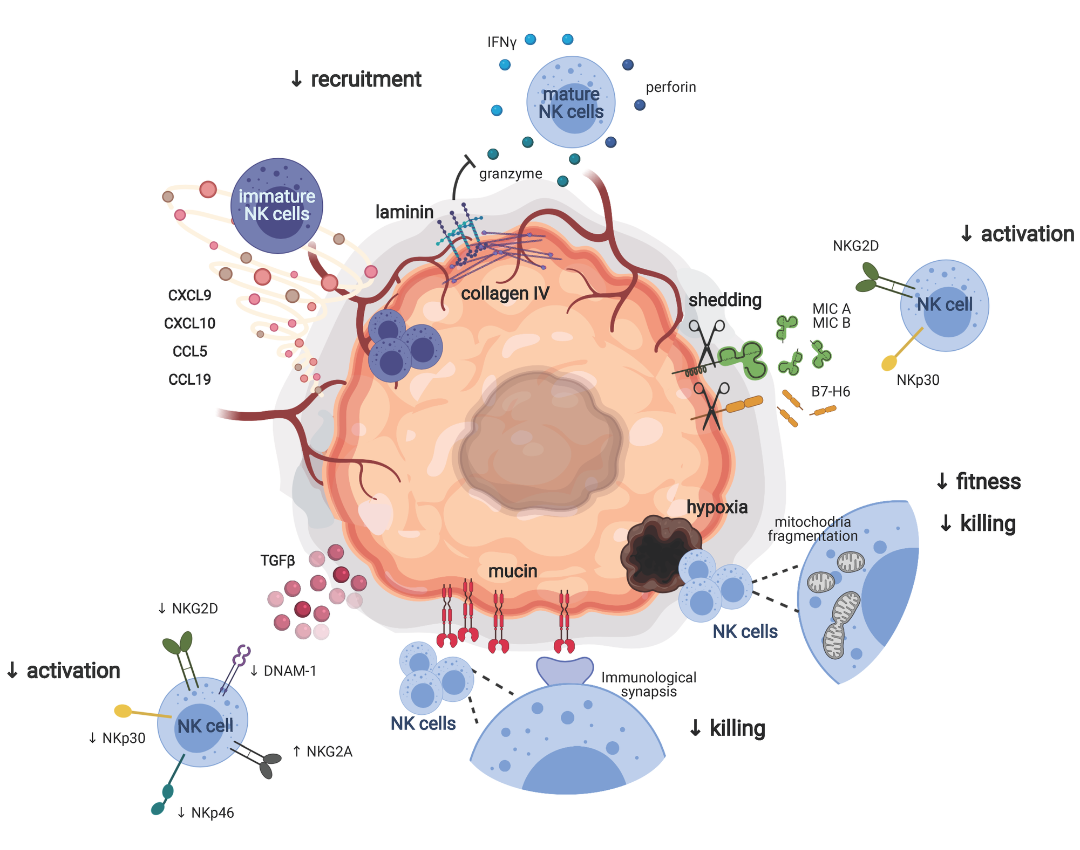
NK cells originate from CD34+ hematopoietic stem cells in the bone marrow and disseminate throughout hematopoietic and non-hematopoietic cells, such as liver, kidney, and spleen blood. Analyzing the characteristics and phenotypes of NK cells, their functional heterogeneity within the NK cell population depends on each individual’s developmental stage [7]. At different stages of maturity, NK cells will exhibit different characteristics and functions determined by the expression of specific receptors. CD56 is a significant marker expressed on NK cells and classified according to the function of NK cells. NK cells in the human body can be divided into two types: CD56-bright and CD56-dim, both of which participate in the cancer immune system with specialized functions [8].
NK cells have two major functions: cell cytotoxicity and immune suppression. If not activated early, NK cells identify and destroy other cells by releasing perforin and granzyme [9]. Furthermore, NK cell activation through activating KAR receptors induces the production and release of factors such as TNFα, FasL, leading to TNF-related apoptosis activating apoptotic pathways. As immune cells, NK cells produce a range of cytokines and chemokines, including IFN-γ, interleukins (IL)-10, CCL3, CCL4, CCL5, and lymphotactin, acting as a bridge between innate and adaptive immunity. Multiple NK cell receptors are specific to major histocompatibility complex class I antigens, nearly expressed on all normal cells but absent on diseased or cancerous cells. By recognizing foreign molecules, MHC-I allows NK cells to detect “missing self” by activating their specific receptors and similar receptors with immune globulins. A stronger activating receptor is CD16, activating antibody-dependent cell-mediated cytotoxicity (ADCC) against cancer cells. ADCC is activated when some immune therapies detect antibodies binding them to the development of cancer NK cells, demonstrating the ability to metastasize cancer and proving the role of NK cells in adaptive immunity [10]. The immune globulin receptor family is collectively called natural killer cell receptors because they are involved in detecting and eliminating virus-infected, fungal, and cancerous cells. These receptors directly activate NK cell cytokine production and cell cytotoxicity.
NK Cell Therapy in Cancer Treatment
Cancer immunotherapy has become increasingly common in the past decade, evidenced by the success of immune checkpoint blockade. Some clinical studies have confirmed a close relationship between NK cells and cancer development [11]. Compared with conventional immune therapies, NK cell therapy has its advantages. With MHC-independent antigen recognition, potent antibody-dependent cell cytotoxicity, high safety, and high flexibility in production, NK cell therapy shows immense potential for cancer treatment [12]. However, cancer patients experience a reduction in the quantity and activity of NK cells, and various other factors in the tumor microenvironment affect the function of NK cells. Thus, strategies focused on restoring the functional activity of NK cells are being conducted through clinical trials. Researchers have studied and demonstrated various methods based on the characteristics of NK cells, such as origin and proliferation, targeting efficacy, and cytotoxicity, in order to use NK cells for monotherapy. Moreover, NK cell transfer combined with other agents, including cytokines and antibodies, yields higher treatment outcomes and efficacy [13].
In patients suffering from leukemia undergoing allogeneic stem cell transplantation, NK cells survive and function until T cells complete their function. Circulating NK cells can access tumor sites through lymphocyte-produced chemokines, and their effector function is regulated by imbalanced signaling from surface receptors [5]. NKG2D is a crucial receptor in aiding immune cells to eliminate cancer cells. The stimulatory expression of the NKG2D receptor on NK cells also modulates the cytolytic ability in patients with acute myeloid leukemia (AML), myelodysplastic syndrome, multiple myeloma, and ovarian cancer [14, 15].
Furthermore, ADCC gets activated in response to CD16 recognition. Strategies enhancing ADCC through NK cell intermediates, such as altering Fc segments or antibody engineering, are alternative options, allowing outcome prediction in clinical trials [11, 16]. Preventing the release or coupling of CD16 with target antigens in NK cells is also under investigation. NK cells rapidly synthesize and release perforin and granzyme, initiating programmed cell death. In certain experiments, NK cells act as intermediaries for malignant tumor cell lysis. Despite their limited infiltration and lower cytotoxicity within the tumor microenvironment, NK cells are crucial in combating the oncogenic process. As the progression of cancer correlates with the dysfunction of NK cells, reinforcing their function is essential for anti-cancer immunity [17].
Hence, NK cells are pivotal immune modulators, playing an essential role in cancer immunosurveillance. However, cancer progression diminishes or alters the functions and characteristics of NK cells. NK cell functions are inhibited by various immune suppressive factors, particularly TGFβ. Therefore, numerous studies are being conducted to enhance NK cell anti-cancer functions through cytokines and antibodies that prevent suppression. Nonetheless, technological advancements have facilitated ex vivo generation, expansion, and genetic modification of NK cells, thereby enhancing their anti-cancer properties. The therapeutic efficacy of NK cell therapy, either alone or combined with other agents, has been extensively demonstrated in numerous clinical trials, and further preclinical studies are underway. Thus, it is reasonable to believe that NK cell therapy could be a promising treatment option for cancer.
References:
- https://www.who.int/news-room/fact-sheets/detail/cancer.
- Organization., W.H., WHO report on cancer: setting priorities, investing wisely and providing care for all. 2020.
- Zhao, Y., et al., Editorial: Cancer cell reprogramming: Impact on carcinogenesis and cancer progression. Front Oncol, 2023. 13: p. 1152402.
- Rawla, P., T. Sunkara, and A. Barsouk, Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol, 2019. 14(2): p. 89-103.
- Goff, S.L. and D.N. Danforth, The Role of Immune Cells in Breast Tissue and Immunotherapy for the Treatment of Breast Cancer. Clin Breast Cancer, 2021. 21(1): p. e63-e73.
- Liu, S., et al., NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol, 2021. 14(1): p. 7.
- Stabile, H., et al., Impact of bone marrow-derived signals on NK cell development and functional maturation. Cytokine Growth Factor Rev, 2018. 42: p. 13-19.
- Poznanski, S.M. and A.A. Ashkar, Shining light on the significance of NK cell CD56 brightness. Cell Mol Immunol, 2018. 15(12): p. 1071-1073.
- Balin, S.J., et al., Human antimicrobial cytotoxic T lymphocytes, defined by NK receptors and antimicrobial proteins, kill intracellular bacteria. Sci Immunol, 2018. 3(26).
- Gauthier, M., et al., Natural Killer cells and monoclonal antibodies: Two partners for successful antibody dependent cytotoxicity against tumor cells. Crit Rev Oncol Hematol, 2021. 160: p. 103261.
- Zheng, X., et al., Mitochondrial fragmentation limits NK cell-based tumor immunosurveillance. Nat Immunol, 2019. 20(12): p. 1656-1667.
- Du, Y. and Y. Wei, Therapeutic Potential of Natural Killer Cells in Gastric Cancer. Front Immunol, 2018. 9: p. 3095.
- Ruscetti, M., et al., NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science, 2018. 362(6421): p. 1416-1422.
- Dhar, P. and J.D. Wu, NKG2D and its ligands in cancer. Curr Opin Immunol, 2018. 51: p. 55-61.
- Schmiedel, D. and O. Mandelboim, NKG2D Ligands-Critical Targets for Cancer Immune Escape and Therapy. Front Immunol, 2018. 9: p. 2040.
- Chiossone, L., et al., Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol, 2018. 18(11): p. 671-688.
- Di Vito, C., et al., NK cells to cure cancer. Semin Immunol, 2019. 41: p. 101272.
